QMS Development & Implementation
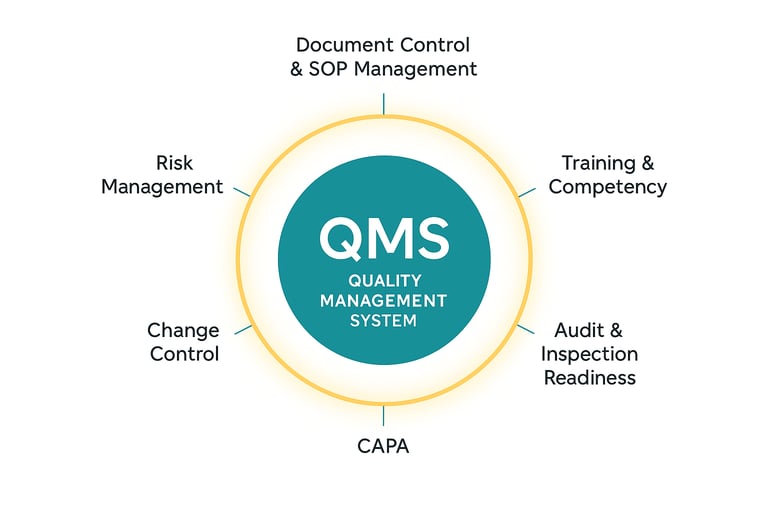
A strong Quality Management System (QMS) forms the backbone of clinical and regulatory excellence. ClinAura designs and implements fit-for-purpose QMS frameworks that help organizations move from compliance-driven activities to a culture of continuous quality improvement. Our approach integrates:
Quality Governance & Leadership — establishing accountability and alignment with organizational goals.
Document & Data Control — ensuring traceable, version-controlled, and ALCOA+ compliant systems.
Risk Management Frameworks — applying risk-based thinking to identify and mitigate issues early.
Deviation, CAPA & Change Management — promoting learning, traceability, and preventive action.
Audit & Self-Inspection Programs — enabling proactive evaluation and inspection readiness.
ClinAura’s QMS solutions are scalable, compliant with ICH-GCP and ISO standards, and tailored to your operational maturity.
→ ClinAura — Building systems that define quality.
SOP & Process Standardization
Standard Operating Procedures (SOPs) are the DNA of operational consistency. At ClinAura, we transform SOPs from static documents into dynamic process enablers that guide performance and ensure global compliance.
Our SOP & Process Standardization services include:
SOP Development and Harmonization — aligned with ICH-GCP, ISO, and regional regulatory frameworks.
Process Mapping and Optimization — identifying gaps and streamlining workflows for efficiency.
Cross-functional Integration — ensuring coordination between Quality, Clinical, PV, and Data teams.
Implementation Support & Training Linkage — connecting procedures to people and performance.
Each SOP is written to be practical, auditable, and fit-for-purpose — ensuring that quality is lived, not just documented.
→ ClinAura — Turning processes into precision.
Training & Continuous Quality Improvement (CQI) Programs
True quality thrives when teams understand it, apply it, and improve upon it. ClinAura’s Training and CQI Programs cultivate a culture of awareness, ownership, and regulatory excellence.
Our programs combine expertise and interactivity to deliver lasting impact:
GxP & Regulatory Compliance Training — foundational and advanced programs across clinical, PV, and QA functions.
Role-Based Competency Development — customized learning paths for operational excellence.
Inspection Readiness Workshops — simulated EMA, FDA, MHRA, and DCGI inspection exercises.
Continuous Quality Improvement Frameworks — performance metrics, CAPA effectiveness checks, and feedback loops.
By empowering people, we strengthen systems and inspire continuous evolution.
→ ClinAura — Empowering people. Elevating quality.