Quality Management Systems (QMS)
The Foundation of Clinical Excellence

At ClinAura, we believe that quality is not a checkpoint — it is a system, a culture, and a commitment woven into every process. A well-designed Quality Management System (QMS) forms the backbone of clinical and regulatory compliance, ensuring every activity is traceable, auditable, and aligned with international standards.
Our approach to QMS development is built on the principles of precision, integrity, and insight, helping organizations transition from compliance-driven operations to a proactive culture of quality excellence.
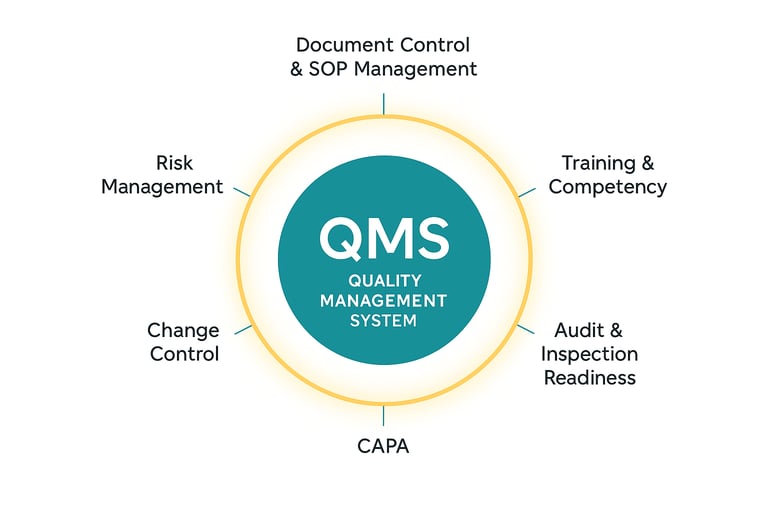
Core Components of a Robust QMS
1. Quality Governance and Leadership
Strong leadership defines the direction of quality. Governance structures establish accountability, oversight, and strategic alignment of quality goals across all operational levels.
2. Document and Data Control
A central pillar of QMS, document control ensures that SOPs, work instructions, and records are accurate, approved, version-controlled, and readily accessible. Proper data management upholds data integrity (ALCOA+) principles.
3. Risk Management Framework
Risk-based thinking drives smarter decisions. Through systematic identification, assessment, and mitigation, potential issues are addressed before they escalate into findings — supporting the principles of Quality by Design (QbD).
4. Training and Competency Management
A QMS is only as strong as the people who implement it. ClinAura emphasizes continuous learning through training programs that build competency, awareness, and accountability across teams.
5. Deviation, CAPA, and Change Management
Effective handling of deviations and change ensures that lessons learned translate into improvement. Our QMS approach integrates CAPA systems that prevent recurrence and strengthen process robustness.
6. Audit and Self-Inspection Program
Internal audits and self-inspections act as the pulse check of quality. Regular, objective assessments help identify trends, ensure readiness for external inspections, and foster continual improvement.
7. Supplier and Vendor Quality Oversight
Ensuring that external partners meet the same standards of excellence is key to maintaining data integrity and patient safety. Vendor qualification and ongoing performance reviews are integral to the QMS framework.
8. Continuous Quality Improvement (CQI)
Quality is a journey, not a milestone. ClinAura’s QMS model embeds CQI practices, using metrics, feedback loops, and performance indicators to drive sustained excellence.
Get in touch
ClinAura operates as a global virtual company, connecting with clients across regions through secure digital collaboration platforms.
Contact us
Service@ClinAura.com


